Former FDA officials and lawyers who advise clients on FDA regulations weigh in on a recent batch of warning letters targeting new dietary ingredients marketed in supplements.
.png?width=850&auto=webp&quality=95&format=jpg&disable=upscale)
FDA in May delivered a batch of warning letters to several companies for selling dietary supplements that “could potentially harm consumers,” according to a constituent update.
FDA targeted six ingredients, including octopamine, higenamine, hordenine, 5-alpha-hydroxy-laxogenin, higenamine HCl and hordenine HCl.
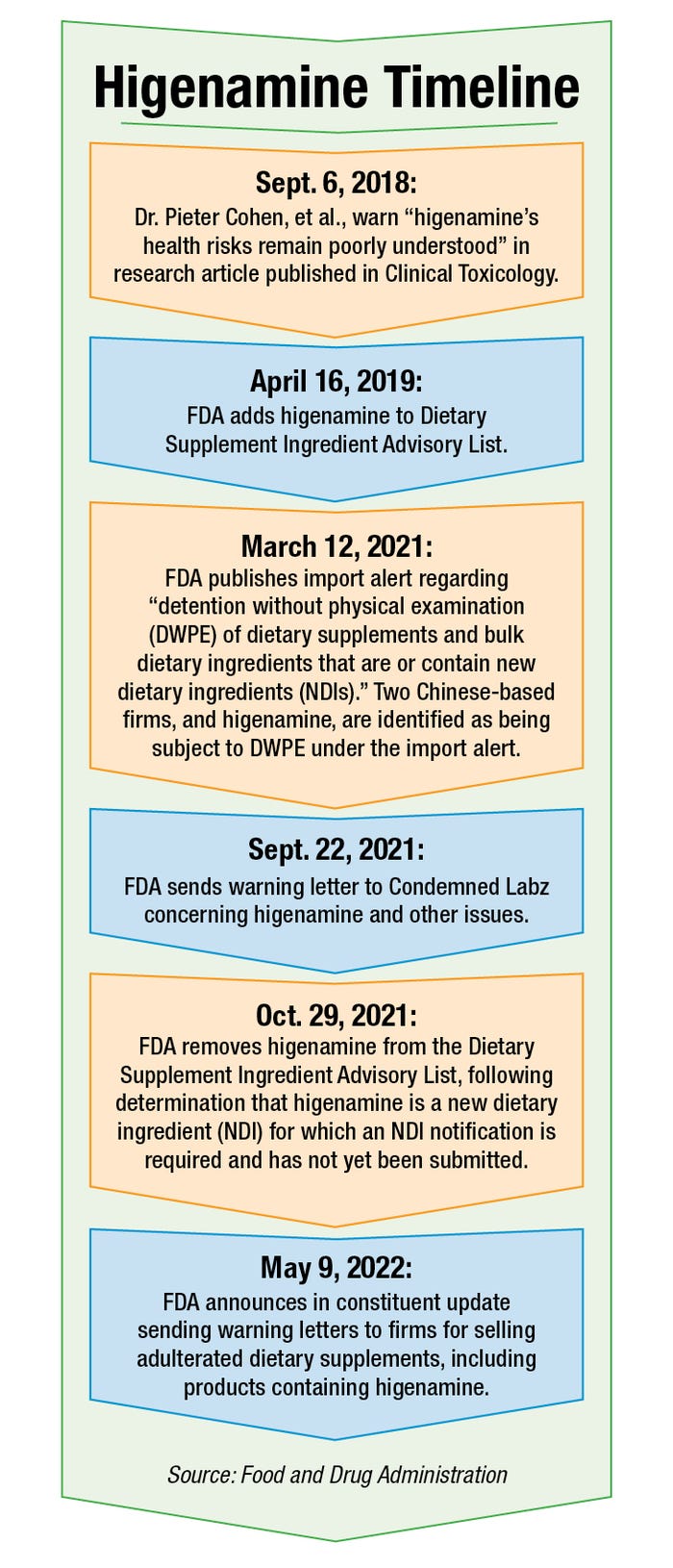
Higenamine is marketed in sports and weight loss supplements. In 2018, in the peer-reviewed medical journal Clinical Toxicology, Dr. Pieter Cohen of Harvard Medical School described higenamine as “a stimulant found in plants” that FDA has never approved as a drug.
“The FDA has received reports of adverse effects from higenamine-containing supplements since 2014, but higenamine’s health risks remain poorly understood,” Cohen and his co-authors wrote in the research article.
Higenamine exerts additional stress on the human heart when injected into a person’s veins, Cohen, an associate professor of medicine at Harvard Medical School, told Natural Products Insider in an interview last year. He said it’s unknown at what dose the ingredient causes such stress when consumed in oral form.
The physician said he wouldn’t be surprised if a low dose of higenamine would be well tolerated and safe, while a high dose would prove to be dangerous.
The problem: “We don’t have the human data to know what’s what,” Cohen explained.
In the recent warning letters, FDA threatened potential legal action, such as an injunction or seizure, if the dietary supplement marketers fail to “adequately address” its concerns. The agency requested companies respond within 15 working days of receipt of the letters.
This is not the first time FDA has raised concerns in warning letters about the likes of higenamine and hordenine, yet it still has not “closed out” some warning letters it sent last year to firms that sold such ingredients. On a webpage, FDA says it may issue a close-out letter once it “has completed an evaluation of corrective actions undertaken by a firm in response to a warning letter.”
Bob Durkin, previously deputy director in FDA’s Office of Dietary Supplement Programs (ODSP), questioned why FDA was sending a second batch of warning letters on the same ingredients rather than taking steps to seize products.
Durkin, who is of counsel in the FDA and health care practices of the law firm Arnall Golden Gregory LLP, cited a “rule of thumb” when he worked at FDA: Don’t send a warning letter unless you are prepared to follow up with legal action, such as a seizure of products or an injunction.
FDA officials maintain “they need more authority, but they’re not using the authority they already have,” Durkin said in an interview. “Go find the stuff in commerce and seize it.”
In the recent warning letters, FDA concluded some of the dietary supplements contain new dietary ingredients (NDIs) subject to a premarket notification, including higenamine, hordenine and octopamine. The agency had not received the required notifications, which FDA officials have described as the agency’s only chance to review the safety of novel ingredients in dietary supplements before they’re marketed to consumers.
In other cases, the products contain “unsafe food additives,” FDA concluded. Those ingredients include 5-alpha-hydroxy-laxogenin, higenamine HCl and hordenine HCl.
One of the warning letters determined some of the products, including CBD gummies, are unapproved new drugs since they are intended to cure, mitigate, treat or prevent a disease.
In requesting responses to its warning letters, FDA said the companies should state “how they will address these issues, or [provide] their reasoning and supporting information as to why they think the products are not in violation of the law,” according to a May 9 constituent update.
NDIs without notifications
In April 2019, an FDA spokesperson said, hordenine and higenamine were added to FDA’s Dietary Supplement Ingredient Advisory List, which “identifies ingredients that do not appear to be lawfully included in products marketed as dietary supplements.” Octopamine was added in June 2019, the spokesperson said.
All three ingredients were removed from the list last year, following FDA determinations that the ingredients are NDIs for which a premarket notification is required but had not been submitted.
Higenamine has not just been flagged in warning letters and the Dietary Supplement Ingredient Advisory List. Companies from China that imported the ingredient into the U.S. have had higenamine detained, according to an FDA import alert covering NDIs “for which there is inadequate information to provide reasonable assurance that such ingredient does not present a significant or unreasonable risk of illness or injury.” Import alert 54-18 suggests higenamine was detained as early as February 2021.
In a Sept. 22, 2021, warning letter to the general counsel of Supplement Science Corp. (doing business as Condemned Labz), FDA described higenamine as an NDI subject to the premarket notification requirement. The agency maintained the ingredient would be adulterated, even if it had received the premarket notification, based on its evaluation of relevant safety information.
“In fact, the available evidence underscores higenamine’s potential for serious cardiotoxic effects,” an FDA official, Ronald Pace, wrote in the warning letter. “In the absence of adequate information to provide reasonable assurance that such ingredient does not present a significant or unreasonable risk of illness or injury, dietary supplements containing higenamine are adulterated under section 402(f)(1)(B) of the” Federal Food, Drug & Cosmetic Act (FDCA).
Hordenine also was the subject of a warning letter sent in October 2021 to Dominant Nutrition LLC. FDA concluded in the letter that hordenine was an NDI that had not received the required notification, rendering the product LipogenX adulterated, and the agency said it was unaware of any evidence to show “hordenine will reasonably be expected to be safe when used as a dietary ingredient.”
FDA has not closed out the warning letters to Condemned Labz and Dominant Nutrition, an FDA spokesperson verified.
According to the Supplement Facts label on Condemned Labz’s website, its product, Convict Stim, still contains 25 mg of higenamine. Meanwhile, nearly all the products listed on Dominant Nutrition’s website—including hordenine-containing LipogenX—are sold out.
Neither Condemned Labz nor Dominant Nutrition immediately responded to requests for comment.
Non-dietary ingredients
FDA described other ingredients in Condemned Labz’s products as “unsafe food additives,” including hordenine HCl and higenamine HCl, which also were mentioned in the May 2022 warning letters. According to FDA, hordenine HCl and higenamine HCl neither qualify as dietary ingredients nor are generally recognized as safe (GRAS) under the conditions of use in the company’s supplements.
“Higenamine HCl is the hydrochloride salt of higenamine and hordenine HCl is the hydrochloride salt of hordenine,” an FDA spokesperson said. “The salt forms are different compounds than the neutral non-salt forms.”
However, it’s possible the ingredients above could qualify as dietary ingredients, according to Durkin, the former FDA official. The scientific question is whether the addition of a salt—namely hydrochloride—constitutes an alteration or modification, he said.
“If the addition of salt is just a modification, it still qualifies as a dietary ingredient,” Durkin explained. “Here, FDA must be saying they have science to show this is an alteration that isn’t reversed upon ingestion.”
FDA, he said, “should share with regulated industry how these specific salt forms constitute chemical alterations and not modifications.”
Plans to reformulate
One of FDA’s recent warning letters was sent to Robert DiMaggio of Ironmag Labs. In a video interview with Jon Bravo Films, DiMaggio said he would reformulate certain products and remove the compounds higenamine and hordenine.
“This isn’t the first time we’ve received an FDA warning letter, and for the record, Ironmag Labs has never been raided,” shared DiMaggio, who pleaded guilty in 2019 to a crime of conspiracy to defraud the U.S. in an unrelated case. “The reason a company will get raided is if you ignore the FDA’s warning letters.”
He cautioned continuing to ignore FDA warning letters could result in an FDA raid and ultimately an indictment charging someone with a federal crime.
FDA “set out new guidelines” and “now it’s companies’ job to follow protocol, period,” Metabolic Nutrition, which received a warning letter from FDA, said in an email to Natural Products Insider. A May 4 letter addressed to Jay Cohen of Nutritional Sales and Customer Service LLC flagged three ingredients marketed in a product called Synedrex: octopamine, hordenine and higenamine. According to the Metabolic Nutrition website, Cohen is a medical doctor who founded the company in 1987.
Other firms targeted in the recent batch of FDA warning letters did not respond to requests for comment for this article.
Industry reaction to warning letters
Several of the warning letters targeting NDIs and non-dietary ingredients did not mention impermissible disease claims. Instead, they were solely focused on the legal status of the ingredients.
FDA’s actions may show the agency’s growing “intolerance to companies” using NDIs without notifying FDA, said regulatory consultant Asa Waldstein, the principal and founder of Supplement Advisory Group, in an interview.
While FDA believes warning letters to companies function as warnings to the broader industry and should deter others, attorney Rick Collins said the letters are often focused on specific marketing claims, products and conduct.
“Other companies will say, ‘Well, we don’t make that particular claim and that’s what attracted the attention,’” observed Collins, a partner with Collins Gann McCloskey & Barry PLLC, who has represented many individuals prosecuted criminally for violating the FDCA. “If Company A gets a warning letter, the principal of Company B will sometimes look at the specific language in the letter and say that those specifics only apply to Company A.”
That FDA is zeroing in on the legality of the ingredients in the recent warning letters is “a step in the right direction” because the specific nature of many warning letters “undermines the broad message that’s trying to be sent,” Collins said in an interview. “The broader the better.”
FDA rarely follows up a warning letter with an enforcement action, such as an injunction, according to attorney Erica Stump in an interview.
That’s partly why she isn’t a fan of federal legislation that would require manufacturers of dietary supplements list their products with FDA. FDA also would be required to create and maintain a database of the listed products under a bill introduced last week in the U.S. Senate.
“We’re going to give the FDA … more stuff to oversee, and they’re not even following up on the stuff that they’re doing right now,” said Stump, a solo practitioner who advises dietary supplement companies.
Amin Talati Wasserman LLP Partner Rend Al-Mondhiry asked a question that many of her colleagues in the legal profession also have raised: “Why does it take so long [for FDA] to take that next step?”
“It does make you wonder why FDA couldn’t use the various enforcement tools it has under the [FDCA] to go after products that appear to pose some pretty significant safety concerns,” she said in an interview.
FDA, DOJ collaboration
FDA provided an emailed statement when asked to comment on criticism discussed in this article.
“A warning letter is the agency’s primary means of achieving prompt voluntary compliance,” an FDA spokesperson said. “We decline to comment on any particular firm or warning letter. As a general matter, the FDA considers a variety of factors in determining whether to refer a particular matter to the Department of Justice [DOJ] to take enforcement action.”
FDA cannot seize products, shut down a firm’s operations through an injunction, or pursue criminal charges without cooperation from DOJ, which brings cases in the federal courts on behalf of FDA.
“It takes a lot more work and justification—and most importantly, the participation of DOJ—to initiate a seizure than to issue a warning letter,” said Ricardo Carvajal, a director with the law firm Hyman, Phelps & McNamara P.C., who previously worked at FDA, where he counseled the agency on rulemaking and enforcement activities and policy initiatives.
Carvajal referenced an FDA Regulatory Procedures Manual (RPM) on judicial actions. A cursory review of the 226-page chapter on judicial actions highlights they require the collaboration of multiple entities within FDA, including at the start of the process.
For instance, after FDA identifies the possibility of executing a seizure or injunction, the RPM recommends the party proposing the action arrange a preliminary assessment call between the Office of Regulatory Affairs (ORA) and other entities in the proposed action, the relevant centers, Division of Enforcement (DE), Office of Chief Counsel, or their relevant designees. DE is located within the Office of Strategic Planning and Operational Policy, which is located within the Office of Partnerships and Operational Policy, which is within ORA.
“Topics may include: the identity of the firm, type of product involved, problems revealed by the inspection, public health risk, jurisdiction and interstate commerce, potential violations of the statute, supporting evidence, relevant compliance policy documents, prior compliance history, scientific support and potential for a corporate-wide action,” the RPM states.
DMAA actions
Dan Fabricant oversaw FDA’s Division of Dietary Supplement Programs from 2011 to 2014. During his tenure, FDA seized products containing the amphetamine derivative DMAA (1,3-dimethylamylamine) after firms continued to sell the ingredient despite earlier warning letters being sent in 2012.
In 2013, FDA administratively detained from USPLabs two DMAA-containing products, OxyElite Pro and Jack3d, following the agency’s efforts to achieve voluntary compliance. FDA that year also seized DMAA-containing products from Hi-Tech Pharmaceuticals. That action led to a years-long battle in the federal district and appellate courts over the legality of DMAA.
FDA had sent several warning letters in 2012 to companies selling DMAA, including USPlabs, though Hi-Tech wasn’t one of the recipients. Ultimately, FDA prevailed in its court fight with Hi-Tech.
Further criticism of FDA
The 2022 warning letters related to higenamine and other NDIs demonstrate “the agency isn’t serious,” according to Fabricant, president and CEO of the Natural Products Association (NPA). “Don’t send a warning letter if you’re not serious about the ending. Step one is the warning letters because we served notice and then step two is … final agency action—whether that be a seizure, [or] whether that be an injunction.”
Asked in an interview whether FDA must hold firms accountable who continue to violate the law, Fabricant responded, “It’s not just that. You have to hold yourself accountable because at the end of the day, the worst thing a regulator can be—and I think we’ve seen it out of FDA for the past four years—is either arbitrary or capricious.”
Attorney Marc Ullman has been critical of an FDA that he described in a 2022 column (published by Natural Products Insider) as “broken,” and he is among FDA’s critics who maintain its enforcement of the NDI notification requirement is lax.
Failure to take the next step beyond a warning letter leaves disagreements with FDA over an ingredient in a state of “purgatory” since firms don’t have “standing” to sue the agency over a warning letter, according to Ullman, who is of counsel to the law firm Rivkin Radler LLP.
“CBD is in this same purgatory and even a more extreme example because on CBD, FDA is on record by saying, ‘We think it’s dangerous and it’s not a lawful ingredient,’” Ullman said in an interview.
In the recent letter to Ironmag Labs, FDA reiterated its long-held position that CBD doesn’t meet the definition of a dietary supplement, and the agency alleged the company’s CBD gummies were unapproved new drugs based on website claims.
FDA’s failure to follow up with enforcement action, Ullman concluded, is “a disservice to the public health and to the trade, FDA’s constituency.”
Collins said it was difficult to push back on some of the criticisms of FDA, which can only send “so many warnings” before it must take further action.
The agency “may prioritize other matters,” the lawyer acknowledged, “but there is a loss of credibility when all you do is keep warning.”
About the Author(s)
You May Also Like






.png?width=800&auto=webp&quality=80&disable=upscale)